Pharma Market Access in Israel.
your product. our efforts.
Registration
Overview
The MoH has an overarching regulatory and policy making role. Foreign companies interested in exporting medical devices or pharmaceuticals to Israel need to appoint a local distributor, agent or other legal representative to register their products. The device registration application should be accompanied by a 510(k), Pre-Market Approval (PMA) or an Investigational Device Exemption (IDE). Pharma registration is more complex and requires additional testing and clinical investigation on top of the product file and FDA/EMA certification. The average time to obtain a market approval from the Ministry of Health is 150 days for medical devices and about 360 days for pharmaceuticals.
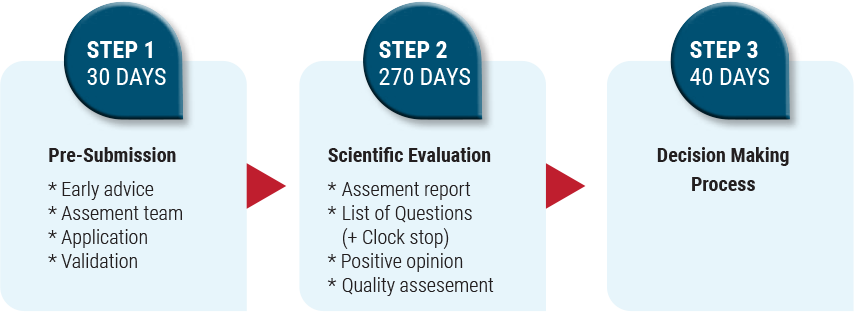
Registration Process In Israel
Registration in Israel follows the registration in the EMA, FDA, Japan and Australia. Complete registration and receing MA in Israel will take less than 1 year. Technology can be filed for registration in Israel immediately after FDA/EMA/Japan/Australia approval.
Technical Standards
Procurement & Tenders
The MoH, its group purchasing organization (SAREL), and the four HMOs publish procurement requests and tenders on their websites. Tenders are published in the Hebrew language. U.S. companies that wish to participate would be required to engage with an Israeli partner.
Ministry of Health current tenders
Regulations
Medical Devices, including biologics that fall under a Biologics Certificate (BK) process at the USA Food and Drug Administration (FDA) Center for Biologics, must be registered with Israel’s MoH Medical Device Registry before they can be marketed in the country. Companies wishing to export medical equipment or devices to Israel must have a local Israeli agent or distributor who should request a pre-marketing approval from the MoH. The request should be accompanied by one of the following documents: the U.S. FDA 510(k), or Pre-Market Approval (PMA). Medical devices fall under medical device classification and require FDA’s BK. In most cases CE and Canadian documentation are also acceptable. If a product is approved by the FDA, it can be registered with the MoH with no further testing. However, electro-medical devices must be tested at the Standard Institute of Israel (SII) for safety and for compliance with the Israeli electric system. In most cases the SII does not accept certifications from other countries.
For any imported medical device, the Israeli importer/agent must submit a registration application to MoH, Department of Medical Devices. The application should include (if available) a certificate issued by a competent authority of one of the following countries: Australia, Canada, European Community (CE) Member States (MSs), Japan, or the U.S. If such a certificate is not available, the registration process may still be available in some cases (usually for lower regulatory class devices) but it will take longer, between two and six months, and the MoH will determine what type of testing is needed.
Product registration supported by existing FDA/EMA documentation usually takes approximately four months. The application for registration of a medical device shall be submitted to the Department of Medical Devices at the MoH. The application should be submitted on the special form designated for this purpose and include the following:
- Name and address of the manufacturer, and of the importer as applicable.
- Description of the intended use of the medical device and of its medical indications.
- Technical details of the medical device and of its components, and if the device or the components are not new, information should be provided on the date of renovation.
- Certificate attesting to the safety of the device, issued by a competent authority of one of the following countries: Australia, Canada, European Community (EC), Member States (MSs), Israel, Japan, USA
- Information on any risk which may be associated with the use of the device (including precautionary measures to be taken).
- Instructions for use of the device in Hebrew; the MOH may allow the instructions to be in English for certain devices.
- Details of the standards to which the device complies.
- Description of the technical and maintenance services, including periodic checks and inspections.
- Declaration, as appropriate: of the local manufacturer/importer, and of the foreign manufacturer.
Note: A license (marketing authorization) for a medical device, granted by the MoH, is valid for up to five years from the date of registration of the device, except for implants with a life-supporting function, for which the validity is for two years from the date of registration.
In addition to the usual requirements for the registration of medical devices, as applicable to the device, specific requirements exist for the products detailed hereunder, which should be included with the application:
- Tissues, including corneas, for transplantation into human beings
- Medical devices containing components derived from animal origin
- Kits for diagnosis of HIV infection
- Bare metal as well as Drug Eluting Coronary stents
Registration costs the equivalent in ILS of $300 per single item, and $1,500 per catalog registration. Catalog registration is, for example, on an EKG device with different channels and accessories. Registration fees are updated by the MoH twice a year. Medical devices are classified according to FDA guidelines.
Technical Standards
The Standard Institute of Israel (SII) is the agency responsible for the development of most product standards, compliance testing, and certification of products and industry quality assurance systems. Israel has stated its intention to follow international standards whenever possible. However, it has been reported that some standards still exist that tend to favour domestic producers over foreign manufacturers. Medical devices must comply with Israel’s electric standard of 220 V, 50 Hz.
Labelling
Medical devices must have Hebrew labelling stating the country of origin, name and address or the manufacturer, and name and address of the Israeli importer. Biological materials should additionally be labelled with their content, weight, and volume in metric units. Implantable medical devices require mandatory labelling in the patient’s file. The labels must contain the following information:
- Name and address of the manufacturer
- Name and address of the importer
- Type of implant
- Sub-type of implant
- Size
- Serial number
- Batch number
- Reused implant
- Medication element needed
- S. Israel Free Trade Agreement & Customs Evaluation
© 2022 ALL RIGHTS RESREVED
Copyright by BWeinreb Medical Market Access Consulting

